Knee Implants
Complex, advanced
knee implants
250K femoral knee systems annually.
An advanced range of proven manufacturing processes for the production of knee systems, including femoral, patella, tibial and limb salvage components.
Our extensive range of capabilities enables us to produce over 250,000 complex femoral knee systems annually with exceptional quality, consistent delivery, and super-cost efficiency.

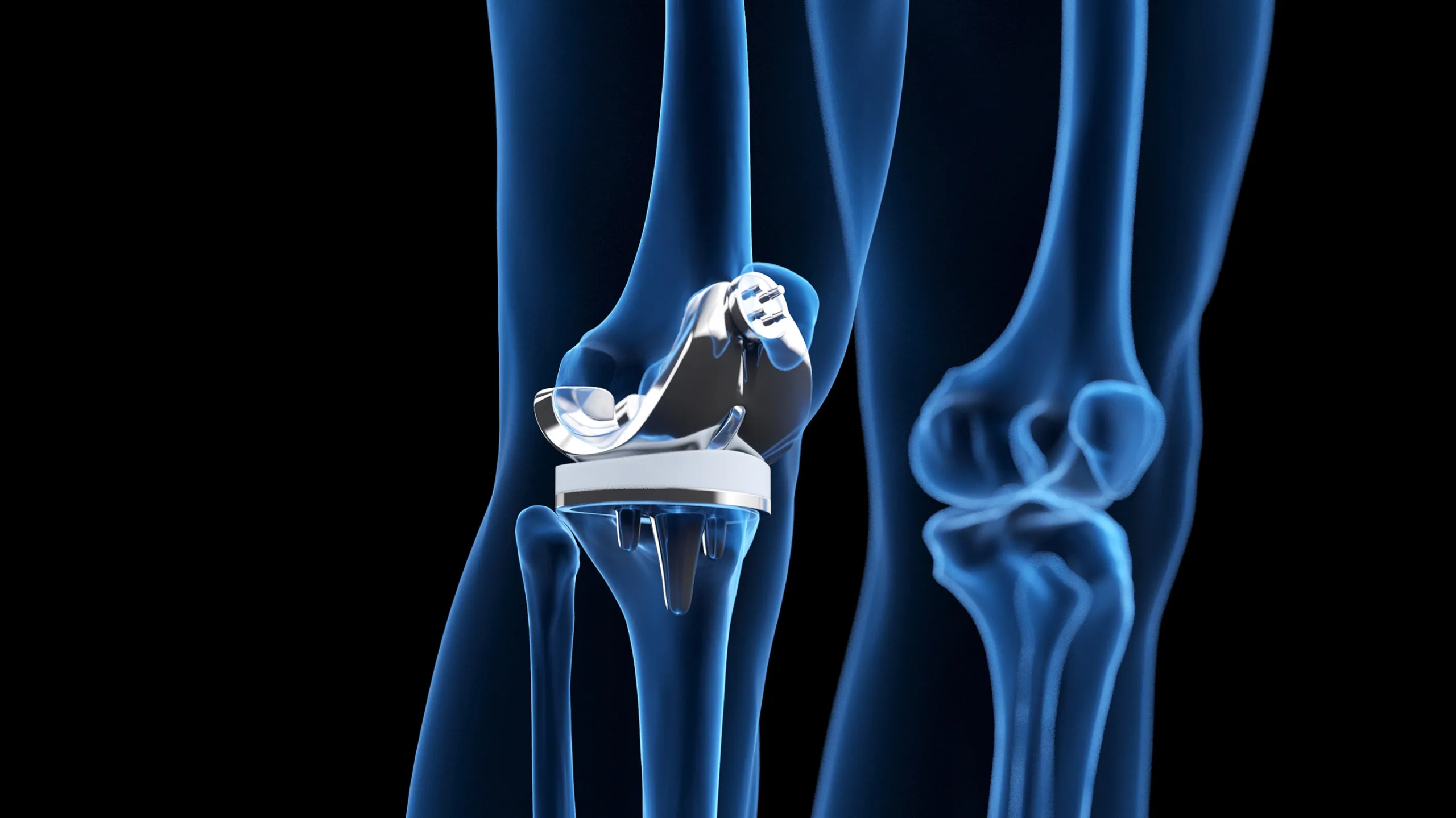
Femoral Implants
Highly precise, innovative solutions
Advanced manufacturing capabilities including automated 5-axis grinding, CNC machining, robotic surface finishing, and laser marking enable us to produce femoral knee implants of exceptional quality and precision. Our commitment to innovation, quality, and exceptional customer service makes us the ideal partner for OEM customers seeking high-quality, reliable, and innovative femoral knee implant solutions.

Patella & Tibia Implants
Complex implants to the highest standards
Our expert team have deep technical knowledge and a proven track record in the manufacturing of patella and tibia implants. Our cutting-edge CNC machining capabilities enable us to create highly precise and complex implants that meet the most demanding standards of the medical industry. With advanced NDT inspection capabilities, we can detect any defects and imperfections, ensuring that our OEM customers receive only the highest quality products.

Limb Salvage
Optimal fit and functionality
Our modular implant solutions are designed to meet the unique needs of orthopedic surgeons treating patients in need of extensive bone reconstruction. Leveraging cutting-edge machining capabilities, we deliver super-precise implants for optimal fit and functionality. Advanced surface finishing, media blasting, and laser marking technologies ensure the efficient and cost-effective preparation of our implants for further processing.
Manufacturing Capabilities
Leverage our agility, ingenuity, automation and low-cost base to drive down your cost of manufacture. We foster a culture of curiosity and exploration, continually invest in the latest technologies and closely collaborate with our customers to drive sustained material and process innovation. Our capabilities and machinery include the following:
Manufacturing Technologies
- DMLS/SLS Additive Manufacturing
- 5-axis CNC Milling & Grinding
- 12-axis CNC Swiss Auto-Turning
- 3-axis CNC Surface Grinding
- Wire & Sinker EDM (Electro Discharge Machining)
- ECM (Electro Chemical Machining)
- CNC Laser Cutting and Welding
- Photochemical Etching
- Welding
Cleaning & Finishing
- Skilled Hand Polishing
- Mass Media Finishing / Drag Finishing (Ceramic, Plastic and Nutshell)
- Robotic Polishing
- Media Blasting
- Stoning
- Automated Electro Polishing
- Shot Peening & Bead Blasting
- Deburring
- Heat Treatment
- CNC Laser Marking
- Surface Texturing and Patterning
- Automated ultrasonic cleaning
Quality Control
- Non-destructive Testing: Fluorescent Penetrant Inspection (FPI) Line
- CMM
- SEM
- X-Ray
- Optical Gauging Products (OGP)
- Visual Inspection
- Hardness Testing
- Mechanical Testing – tensile, compression, and fatigue testing
- Surface Roughness and Finishing Inspection
- Vision Systems
- Software Development Leveraging Artificial Intelligence (AI)
Committed To Excellence
Croom Medical is committed to maintaining an effective quality management system to ensure compliance with all applicable quality and regulatory requirements, through continual improvement. We share a commitment to long lasting relationships based on trust, by partnering with our customers.
Fully Certified
DEVICES
Extend capacity & advance quality
Tap into our passion for innovation and we’ll help advance quality, extend your capacity and accelerate time to market.