
Additive Manufacturing
End-to-end additive
manufacturing
A complete one-stop-shop serving the world’s leading orthopedic innovators. We have the expertise, technologies, scale and equipment to strengthen and accelerate your additive manufacturing journey.
Producing 50K+ high-precision spine implants annually.
Extensive 3-D Printing Capability & Capacity
Validated Laser-Powder-Bed-Fusion technologies from Renishaw, GE and 3D Systems produce high-precision implants. Delivering complex geometries with exceptional accuracy, quality, and consistency.
FDA 510(k) Regulatory Guidance
Regulatory guidance for 510(k) submission process including traditional, abbreviated, and special 510(k) submissions, as well as pre-submission meetings with the FDA. We can also assist with post-approval compliance and ongoing regulatory support.
On-Site Materials Testing Lab
A fully-equipped lab on-site with mechanical, morphological and chemistry testing capabilities. Staffed by experienced PhD researchers and engineers, ensuring that every product is safe, effective and reliable.
World-Class Post-Processing Capability
Not only do we deliver comprehensive AM solutions, but we also provide all your post-processing needs such as EDM, CNC machining, surface finishing, cleaning, heat treatment and HIP. Tailored process steps designed to meet your specific requirements and the rigorous standards of the orthopedic implant industry.
Pushing the Boundaries
For 10+ years, we’ve strategically leveraged industrial and academic partnerships, and active participation in EU Research Consortiums to drive innovation and business success. Such collaborations have been instrumental in enhancing our knowledge of AM, expanding our network, and accessing vital resources so we can pass the benefits to our valued customers.
Advanced Heat Treatments
Our AM process is supported by advanced vacuum and hot isostatic pressing (HIP) technology. Our HIP process involves applying high temperature and pressure to our implants, eliminating internal voids and enhancing their structural integrity.
Additive Innovation
An expert team, precise processes, best-in-class technology. Validated Laser-Powder-Bed-Fusion technologies, featuring a variety of materials, all assigned to individual 3D printers to mitigate against the risk of contamination.
Key Expertise & Capabilities
- Design and engineering support
- FDA 510(k) regulatory guidance
- Computer-aided design services
- Expert prototype development
- Material testing lab
- Serial implant and custom implant production
- Validated finishing processes and post-processing processes
Material Expertise
- Tantalum (Ta)
- Titanium (Ti)
- Stainless steel (Fe-Cr-Ni-Mo)
- Cobalt-chromium (Co-Cr)
Validated AM Technologies
- Renishaw RenAM 500S
- GE ConceptLaser MLab
- GE ConceptLaser M2
- 3D Systems DMP Flex 350 Dual

Osteoconductive & Graded Structures
In addition to co-development and design of orthopedic implants, Croom Medical offers fully customised lattice structures with exceptional osteoconductive and graded structures. Backed by our teams extensive expertise in Surface Engineering, we excel in precision techniques such as etching, coating and leveraging laser-powder-bed-fusion (3D printing) technologies.

Pioneering Tantalum Material Development
In 2022, Croom Medical’s RD&I team identified a market need for innovative orthopedic joint replacement materials. We recognised that Tantalum metal offers superior characteristics compared to traditional Titanium, including enhanced stability and longevity of joint replacement implants.
To explore the full potential of tantalum and capitalise on its superior qualities, we forged a collaboration with Global Advanced Metals (GAM) to establish a cutting-edge Ta-based medical device additive manufacturing capability using Laser Powder Bed Fusion technologies. Croom Medical’s deep expertise in additive manufacturing combined with GAM’s high-quality tantalum powder ensures that unique structures and devices can be created that exhibit exceptional performance.
Recently, we launched our first hybrid Titanium and Tantalum-based orthopedic implant prototype positioning ourselves at the leading edge of orthopedics manufacturing globally.
Collaborations & Partnerships
Our Innovation Partners










Sparking innovation
At Croom Medical we foster a culture of curiosity and exploration, continually invest in the latest technologies and closely collaborate with our customers to drive sustained material and process innovation.
- Dedicated, in-house automation team reduce costs & increase quality.
- €12M invested since 2019 to advance our manufacturing capabilities.
- Real-time reporting for transparent customer communications.
- Croom Medical’s industry 4.0 strategy ensures that manufacturing equipment is directly linked to our ERP system where live OEE, PM and SPC analysis can be openly viewed at any time. Customers can receive automated reports twice daily, providing complete transparency on manufacturing progress.
- Using the latest material technologies, including Tantalum.
- In 2022, our RD&I team identified a need for innovative orthopedic joint replacement materials, recognising Tantalum’s superior qualities over Titanium. To harness Tantalum’s potential, Croom Medical has collaborated with Global Advanced Metals (GAM) to establish a cutting-edge additive manufacturing capability using Laser Powder Bed Fusion (3D Printing). Recently, Croom Medical launched its first hybrid Ti-Ta based orthopedic implant prototype.
- Leveraging close partnerships and collaborations.
- For more than a decade, we’ve strategically leveraged industrial and academic partnerships, and actively participated in EU Research Consortiums to drive innovation and business success.
- Award-winning Research, Development & Innovation team
- MedTech Partner/Supplier of the Year 2023, Irish MedTech Awards
- Innovation Excellence Business Award 2023 Finalist, Limerick Chamber
- Best Overall Business of the Year 2021, Limerick Chamber
- Best SME Business: Contribution to the Region 2021, Limerick Chamber
- Contract Manufacturer of the Year 2019, IMR Supplier Award
- Advanced Manufacturing Award 2018, IBEC
- Innovation Award 2017, Enterprise Ireland
- Innovative Project of the Year 2013, Engineers Ireland
- Innovative Project of the Year 2011, Engineers Ireland
- Stryker Supplier of the Year 2007

Our Facility
The scale to consistently deliver
24/7 Production
A growing team of 150+ product builders, engineers and professionals delivering top quality, reliable medical devices.
Certified
Certified to ISO 13485 and 14001 standards. FDA registered facility
Latest, Cutting-Edge Equipment
More than €12M invested in plant & machinery over the past 4 years to advance our capabilities.
On-Site RD&I Lab
We operate our own on-site R&D and materials testing lab for tensile, compression and fatigue testing.
Labour Knowledge & Skills
Situated in Limerick, the centre of Ireland’s MedTech manufacturing cluster with a wealth of labour knowledge and skills in orthopedic manufacturing.
Quality-At-Scale
Producing quality at volume, with 3.5M+ femorals manufactured to date. And we’re growing, with floor space increasing by more than 10,000 sq. feet in 2024.
DEVICES
Extend capacity & advance quality
Tap into our passion for innovation and we’ll help advance quality, extend your capacity and accelerate time to market.
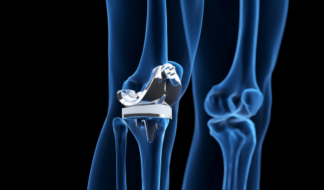
Knee Implants
Femoral implants, patella and tibia implants, and limb salvage.
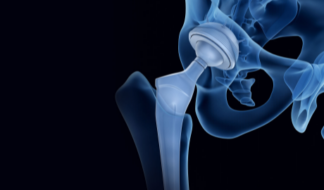
Hip Implants
Hip stems, acetabular cups, femoral heads and femoral liners.
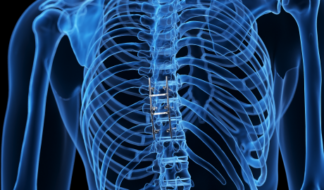
Spine Implants
Interbody cages, pedicle screws and rods, and SI fixation screws.
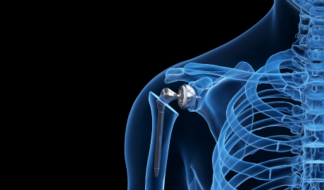
Trauma & Extremities
Shoulder & elbow, foot & ankle, tibia & femur, hand & wrist, cranial & maxofacial.
Capabilities
Joint Partnership
We help drive progress for our medical device customers by enhancing both their production capacity and their product and process innovation.
Manufacturing Capabilities
The facilities, technologies and processes to deliver end-to-end manufacturing.
Value-Add Capabilities
The team, expertise, and ingenuity to drive tangible added-value for our customers.