Watch the webinar on-demand here: https://youtu.be/-PIIkfxG5XU?si=uogjrdcvCMHATApy
Stay Ahead of the Curve in Orthopedic Innovation with Croom Medical’s Upcoming Webinar in collaboration with Global Advanced Metals (GAM).
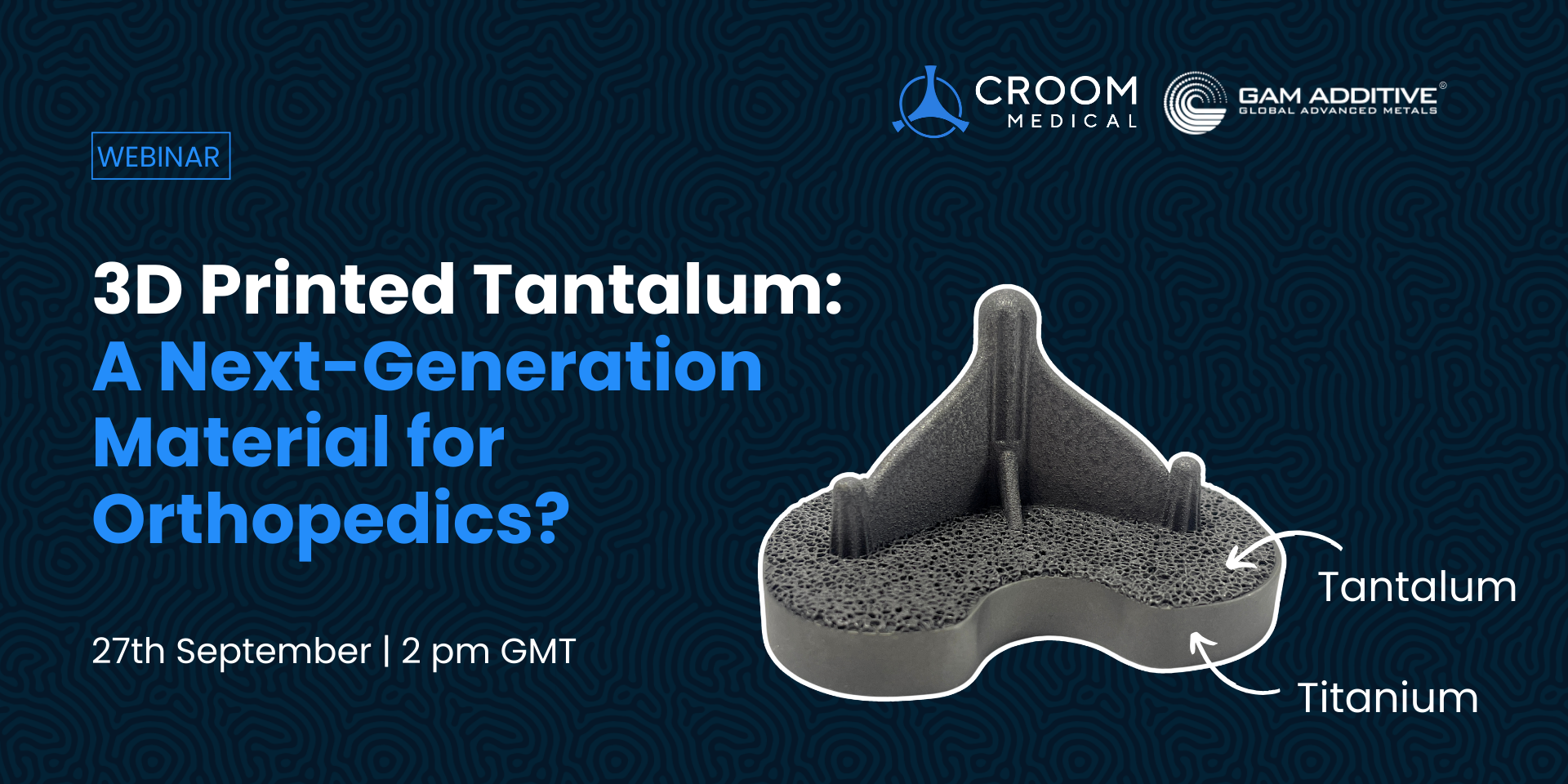
Croom Medical is proud to announce an upcoming webinar in collaboration with GAM that will delve into the exciting realm of orthopedic materials and explores the remarkable potential of 3D Printed Tantalum (Ta) as the next-generation material for orthopedic implants.
The webinar, which takes place on Wednesday 27th September 2023 at 2 pm GMT, promises to be an insightful session that will delve into the fascinating realm of additive manufacturing in orthopedics. Participants will have the opportunity to explore the cutting-edge Ta-based demonstration device designs and gain valuable insights from the initial testing data of tantalum parts manufactured using GAM’s high-purity powders. This wealth of information is set to provide attendees with a comprehensive understanding of the remarkable advancements made in the field and the boundless possibilities that lie ahead in the realm of orthopedic implants.
About Tantalum
Tantalum has been the subject of numerous in vivo and in vitro studies, demonstrating exceptional properties such as biocompatibility, corrosion resistance, osseointegration, and high ductility. These properties make tantalum devices attractive for orthopedic applications, offering significant advantages such as preventing bone resorption, reducing stress shielding, and minimizing implant loosening.
Croom Medical’s Partnership with Global Advanced Metals
To explore the full potential of tantalum and capitalise on its superior qualities, Croom Medical has forged a partnership with Global Advanced Metals (GAM). Together, they have established a cutting-edge Ta-based medical device additive manufacturing capability. Through this collaboration, they have achieved the creation of unique structures and devices, showcasing promising performance results. Importantly, this partnership has enabled the industrial-scale production of intricate shapes and structures, making tantalum implants a viable reality for patients in need.
Want to learn more? Watch the webinar on demand here: https://youtu.be/-PIIkfxG5XU?si=uogjrdcvCMHATApy
About the Presenters
Patrick Byrnes, CEO, Croom Medical
Patrick Byrnes is the visionary CEO of Croom Medical since 2018, leading a team of 180+ skilled professionals in manufacturing orthopedic implants for top medical device companies. With over 15 years of experience in the orthopedic medical device industry, Patrick’s expertise has driven the company to a remarkable threefold sales growth, maintaining an impressive upward trajectory. Armed with a Bachelor of Engineering from Munster Technological University (MTU) and a Master’s in International Business Management from University College Dublin (UCD), Patrick’s commitment to excellence is evident. He further honed his business acumen with Diplomas in Business Development from Queen’s University Belfast (QUB) and International Selling from Trinity College Dublin (TCD). Recognized for his R&D developments, he has earned prestigious awards like Engineers Ireland Student of the Year (2011 and 2013) and Enterprise Ireland’s Innovation of the Year (2016).
Dr. Shane Keaveney, R&D Manager, Croom Medical
Dr. Shane Keaveney is an accomplished expert in Digital Manufacturing and Additive Manufacturing, holding a Ph.D. in kinematic error modeling of machine tools for manufacturing processes. With over a decade of academic and industrial experience, he serves as the Research & Development Manager at Croom Medical, driving innovation and leading projects funded through national and European sources. Shane’s exceptional contributions include multiple published papers and a patent for a groundbreaking 3D-printed biomaterial. His role involves coordinating research and deploying industry 4.0 technologies to advance Metal Additive Manufacturing for Medical Devices, ultimately enhancing quality, and reducing costs. Alongside his pivotal role at Croom, Shane lectures material science and design for manufacturing in the Department of Design Innovation at Maynooth University.
Gordon Smith, Chief Technical Officer, Global Advanced Metals
Gordon Smith holds a Bachelor of Science degree in Chemical Engineering from Michigan Technological University, a Master of Science degree and a Doctorate of Science degree in Chemical Engineering both from the Massachusetts Institute of Technology, and a Master of Business Administration with a concentration in Finance from the University of Chicago. He has led the R&D organization at GAM since 2019, leveraging over 25 years of experience in developing product solutions in electronic materials, stereolithography resins, and medical devices.
About Croom Medical
Croom Medical is a high-tech award-winning contract manufacturer with 39 years of experience, specialises in joint replacement orthopedic implants and medical devices. Our innovative R&D team and 170+ staff support numerous blue-chip clients. Producing 230,000+ femoral knee systems annually, our state-of-the-art FDA-registered and ISO 13485-certified facility utilizes advanced machining and additive manufacturing process technologies. Partner with Croom Medical for outstanding expertise and dedication to excellence in medical manufacturing. Your success is our mission.
About Global Advanced Metals
A leading conflict-free tantalum producer, GAM has exclusive rights to the world’s largest industrial resources of tantalum ore located in Western Australia where GAM extracts tantalum as a co-product of lithium mining. GAM produces conflict-free tantalum powders and metallurgical products at its Pennsylvania, USA, and Aizu, Japan plants for a range of industries including electronics, aerospace, defense and medical. GAM’s facilities in Japan and the USA were the first to be declared “Conflict Free” in 2010 under the, now called, Responsible Minerals Assurance Program and have maintained conformance to this program every year since.
