The journey of material use in orthopedic implants has been a fascinating interplay of engineering, material science, and medical innovation. As the demands of patient care continue to evolve, so do the materials and techniques used to craft these life-changing devices. In this blog post, we explore the historical milestones of bone interface materials and future potential of materials such as Tantalum in medical devices.
Early Materials: Stainless Steel and Vitallium
In the early 20th century, stainless steel and Vitallium (a cobalt-chromium alloy) dominated the orthopedic implant scene. These materials were chosen for their impressive strength and corrosion resistance. However, fixation methods at the time relied on mechanical fixation or bone cement, often leading to stress shielding and eventual loosening of the implants. Despite their durability, these early materials lacked a focus on biological integration, leaving much room for innovation.
The Introduction of Titanium: A Game-Changer in Osseointegration
An unexpected discovery by Per-Ingvar Brånemark in 1964 transformed implant technology. While studying microcirculation in rabbits, he observed that the titanium camera he used to monitor blood flow had fused with the bone tissue. This unexpected phenomenon, which he later termed “osseointegration,” became the foundation for modern implantology.
This groundbreaking insight positioned titanium as a preferred material for orthopedic implants. With its excellent strength-to-weight ratio, biocompatibility, and corrosion resistance, titanium raised the bar for implant design, minimising adverse reactions and establishing a new standard in the field.
Advancements in Surface Modifications: Bridging Mechanical and Biological Fixation
While titanium provided the foundation, researchers sought to enhance osseointegration further through implant surface modifications. Techniques such as porous metal beads and wire meshes sintered onto cobalt-chromium implants created scaffolds for bone ingrowth, offering stronger and more reliable fixation.
Hydroxyapatite (HA) coatings were another leap forward. By mimicking the mineral composition of natural bone, HA promoted direct bone attachment, improving biological integration. Surface roughening techniques like grit blasting and plasma spraying increased surface area and mechanical interlocking capabilities.
These innovations marked a critical step in transitioning from purely mechanical fixation to true biological integration.

Titanium femoral components with porous structure 3D-printed on the Renishaw AM500S machine at Croom Medical’s state-of-the-art Additive Manufacturing Centre
Additive Manufacturing: Redefining Implant Design
The introduction of additive manufacturing (AM) marked the beginning of a new chapter in orthopedic implants. AM technologies enabled the creation of porous titanium structures that closely mimicked natural bone, fostering more natural and secure fixation through bone ingrowth. The capability to design complex geometries and patient-specific implants further expanded the horizons of orthopedic care. These tailored solutions not only improved surgical outcomes but also enhanced patient satisfaction.
Check out our recent blog post on ‘7 Key Implant Design Considerations for Additive Manufacturing’ to see how successful AM requires specific implant design approaches that differ from traditional manufacturing.

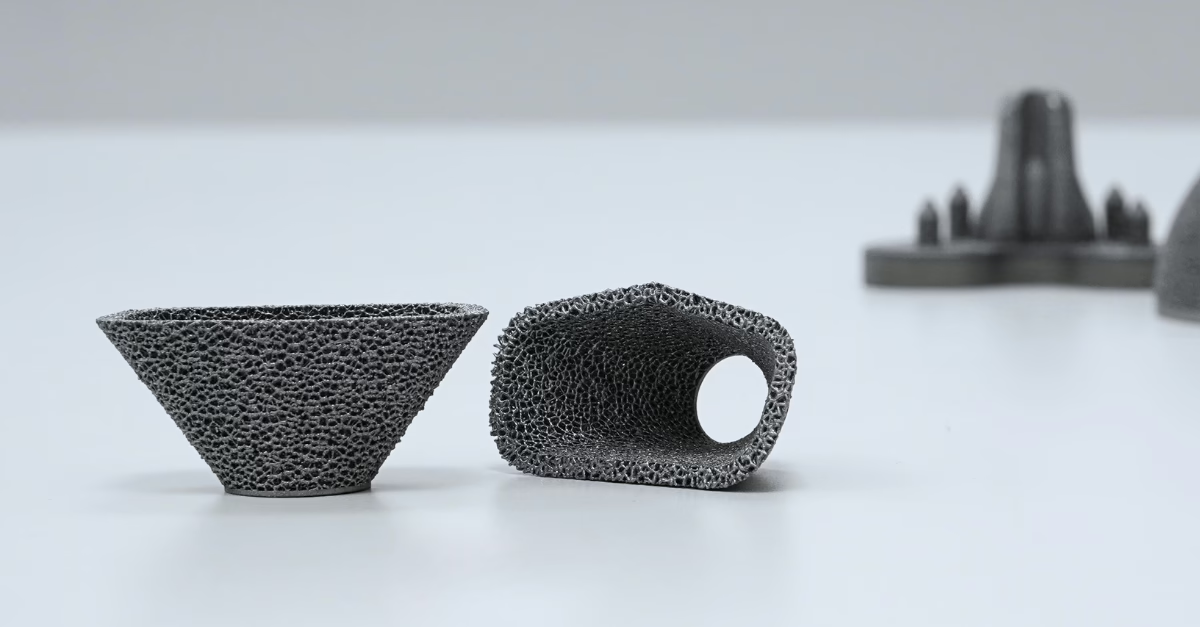
Acetabular cup implant 3D-printed in tantalum material by Croom Medical’s award-winning R&D department.
The Rise of Tantalum: A New Frontier in Bone-Interface Materials?
Today, tantalum is emerging as a transformative material in orthopedic implant technology. Its unique properties—including biocompatibility, corrosion resistance, and mechanical properties make it an ideal material for supporting bone growth and integration in implants. Reducing the risks of toxicity, bone resorption, and implant loosening due to stress shielding.
While Tantalum is hard-to-process it is particularly well-suited for additive manufacturing. Research led by Bandyopadhyay et al., published in Additive Manufacturing, highlights that in a direct comparison additively manufactured porous tantalum outperforms titanium in interfacial integration, enabling faster bone ingrowth and shorter healing times. However, the use of tantalum in metal additive manufacturing is relatively new.
Through a strategic collaboration between Croom Medical and Global Advanced Metals, a cutting-edge platform has been developed to produce bone-like porous tantalum using advanced AM techniques. This innovation facilitates the creation of full-scale tantalum implants and hybrid multi-material solutions such as tantalum-titanium (Ta-Ti) implants, paving the way for remarkable advancements in implant technology.
Conclusion – Shaping the Future of Orthopedic Implants
As the field of orthopedic implant manufacturing continues to evolve, the integration of innovative materials like tantalum and state-of-the-art techniques such as additive manufacturing is set to redefine patient outcomes. With several current customers already in prototyping phase, Croom Medical’s 3D printing platform for tantalum is now available within its contract manufacturing model.
Are you ready to elevate your next joint replacement implant project? Whether your project involves large-scale production or custom, high-complexity implants, Croom Medical has the expertise and technology to deliver superior results.
Connect with Croom Medical’s highly experienced joint replacement implant manufacturing team for tailored solutions and industry-leading expertise. Contact us today to discuss how we can help bring your project to life.
