Watch the webinar on-demand here: https://youtu.be/0FwEzx2l-38?si=bZMmPqpOPyJuJrSC
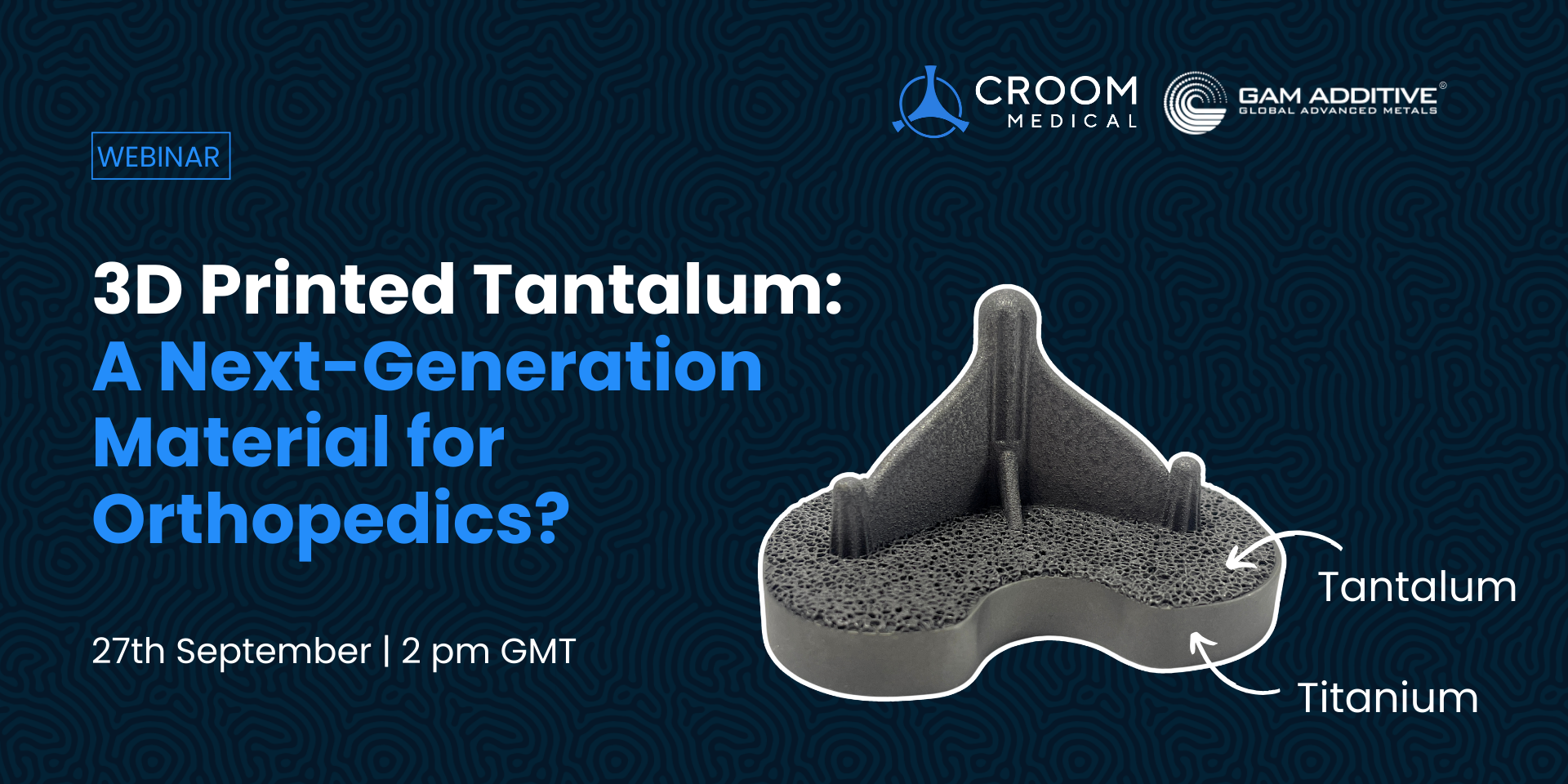
Are you looking to enhance the performance of your 3D-printed titanium implants with state-of-the-art materials science and characterisation techniques?
Join Dr. Bryan Naab, Research Engineer at Croom Medical, for an insightful webinar exploring how advanced materials characterisation combined with mechanical and fatigue testing, is driving the development of high-performance titanium implants – manufactured leveraging metal Additive Manufacturing (AM) technologies.
Discover innovative strategies to optimise material properties, ensure superior implant performance, and stay at the forefront of medical device innovation through the latest advancements in AM processes.
In this webinar, you’ll learn how heat treatments fine-tune mechanical properties through microstructural changes and see how optimising AM process parameters can dramatically improve fatigue strength. Get an inside look into the critical material characteristics that influence the fatigue failure of titanium AM implants.
The session will delve into the following topics:
– Explore how advanced material characterisation techniques like microstructural analysis, µCT-Scanning, and X-ray diffraction are used to develop stronger, more reliable implants with enhanced performance.
– Learn how static mechanical and fatigue testing are used in practice to make process improvements and to select post-processing strategies.
– Understand the pivotal role of controlling defects, surface roughness, and microstructure to not only boost material performance but also reduce costs.
Supported by Dr. Shane Keaveney, R&D Manager at Croom Medical, the webinar highlights Croom Medical’s expertise in metal Additive Manufacturing and Materials Science. This is underpinned by recent investment in a state-of-the-art material testing laboratory and academic collaborations with University of Limerick, Technical University of the Shannon and University College Dublin. Attendees gain practical strategies for identifying and mitigating potential weaknesses in both implant and process design.
Don’t miss this opportunity to deepen your knowledge of the critical material characteristics that influence the fatigue failure of titanium AM implants. Learn how to optimise your additive manufacturing processes for enhanced implant performance.
Speaker(s):
- Dr. Bryan Naab – R&D Engineer at Croom Medical
- Dr. Bryan Naab is a Research Engineer at Croom Medical, specialising in material testing and additive manufacturing. He holds a PhD in Metallurgical Engineering from University College Dublin, where his work focused on fatigue prediction and advanced materials characterisation in additively manufactured Ti-6Al-4V alloys. He has collaborated with one of the world’s leading Med-Tech OEMs and has extensive research experience across sectors, including the development of bio sensors and the processing of ultra-thin glass substrates.
- Dr. Shane Keaveney – R&D Manager at Croom Medical
- Dr. Shane Keaveney is an accomplished expert in Digital Manufacturing and Additive Manufacturing, holding a PhD in kinematic error modelling of machine tools for manufacturing processes. With over a decade of academic and industrial experience, he serves as the Research & Development Manager at Croom Medical, driving innovation and leading projects funded through national and European sources. Shane’s exceptional contributions include multiple published papers and a patent for a groundbreaking 3D printed biomaterial. His role involves coordinating research and deploying from industry 4.0 technologies to advance Metal Additive Manufacturing for Medical Devices, ultimately enhancing quality, and reducing costs. Alongside his pivotal role at Croom, Shane lectures material science and design for manufacturing in the department of Design Innovation at Maynooth University.
- About Croom Medical
- Croom Medical, based in Ireland, is a contract manufacturer with 40+ yrs experience producing high-quality joint replacement implants for leading OEM’s. Leveraging advanced manufacturing technologies and deep industry expertise, Croom Medical helps medical device OEMs accelerate time-to-market, boost production and secure their supply chain. Their FDA-registered & ISO 13485 certified facility is supported by 150+ professionals, including an award-winning Research, Development & Innovation team.